by Lewis I. Held, Jr.
purchasing information
| Quirks of Human Anatomy by Lewis I. Held, Jr. |  purchasing information |
| back to Quirks index page | |
Figure Legends 5 5.1 * 5.1R * 5.2 * 5.2R * 5.3 * 5.3R figure legends 1 * 2 * 3 * 4 * 6 * 7 * A N.B.: An 'R' suffix denotes reflections (commentaries, annotations, and further references) pertaining to the numbered legend that precedes it. [Select any image to enlarge; use back button to return] Fig. 5.1 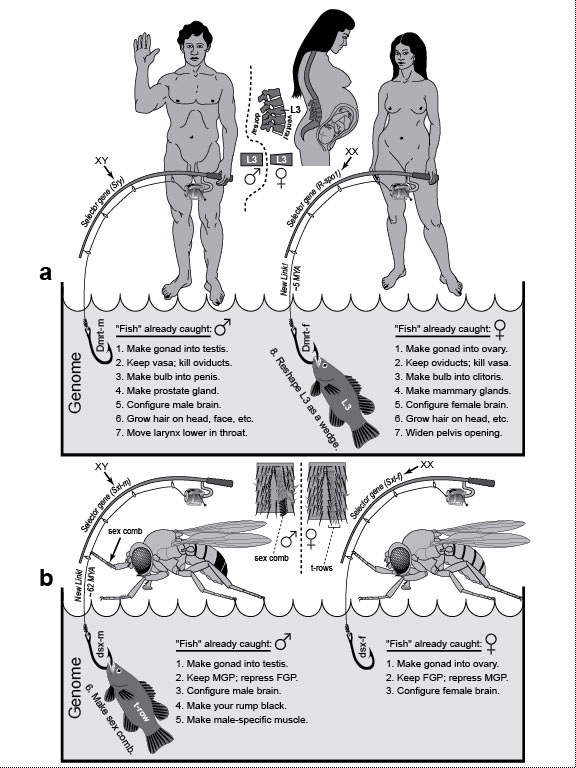 Genetic basis for sexual dimorphisms in humans versus fruit flies. One example per species is used for illustration, with samples of other sex-limited traits listed in "gene pools" below (cf. Fig. 5.2). The fishing theme extends a metaphor begun in Fig. 4.2: fishing poles represent master genes, and the fish symbolize target genes (written here as English imperatives to make specific anatomical traits). Genetic basis for sexual dimorphisms in humans versus fruit flies. One example per species is used for illustration, with samples of other sex-limited traits listed in "gene pools" below (cf. Fig. 5.2). The fishing theme extends a metaphor begun in Fig. 4.2: fishing poles represent master genes, and the fish symbolize target genes (written here as English imperatives to make specific anatomical traits).
a. Human dimorphisms. Men and women differ in various features, including their lumbar vertebrae, a disparity that evolved after our ancestors became bipedal [2795]. A pregnant woman (side view) bends her back (lordosis) more than a man to bear the weight of the baby. Her L1-L5 vertebrae are magnified alongside, with L3 schematized to contrast its shape (wedge) with that of the male L3 (block). The master gene for maleness (under the XY switch) is Sry (Sex-determining region [on the] Y), and it has been known for some time [2056,2756], whereas its presumptive counterpart (under the XX switch [221]), R-spo1 (R-spondin1), was only identified recently [2808]. R-spo1 is a diffusible signal that may use Wnt-pathway transcription factors to control its target genes. R-spo1 is presumably able to reshape L3 because of its having captured a gene(s) that spurs growth in the ventral versus dorsal half of the vertebra. That capture must have occurred ~5 MYA (cartoon below where the fish denotes an eighth trait) because it is seen in Australopithecus africanus [2795]. The proximate "bait" (at the end of the sex determination pathway but before any target genes) may be Dmrt, the human homolog of dsx in flies, which, like dsx, is spliced differently in males and females (suffixes "-m" and "-f") [2899]. Of the 8 putative Dmrt paralogs in humans [1418], only Dmrt1 (on chromosome 9) seems critical [1957], although much remains to be learned about this circuit [221,270,2333]. b. Fly dimorphisms [327]. Interestingly, the species D. melanogaster is named for a dimorphism—namely, the dark (melano-) abdomen (-gaster) in the male. We’ve learned a lot about the genetic basis of this trait lately [1294,1423,2476,2837]. MGP and FGP are male and female genital primordia, respectively (cf. Fig. 5.2g-i). The "sex comb" (so named because it resembles a hair comb and is present in only one sex) is a row of bristles that may help males grip females during mating [1874], although it could be just an ornament [2055]. The cylindrical tarsal segment where it is located is drawn as a panorama (male vs. female) by imaginarily cutting along the dorsal midline and spreading it out flat (proximal above, distal below). Flies are covered with intricate patterns of this sort [1134], but only the sex combs are shown in the whole-body sketches for the sake of clarity. Developmentally, the comb arises as a transverse row (t-row) that rotates ~90 degrees [1138,2567]. Evolutionarily, its bristles became thicker, blunter, darker, and more numerous as it underwent alterations in various lineages [1425]. The taxonomic distribution of sex combs indicates that they originated near the base of the subgenus Sophophora (after its divergence from other subgenera but before the splitting of melanogaster and obscura species groups) [1425] ~62 MYA [103]. Genetically, the sex comb and other dimorphisms depend on a hierarchy of control genes [457,993], beginning with ones that count the number of X chromosomes (sic, not the X:A ratio!) [727]. On the basis of that number, the master switch Sex-lethal [1576] gets spliced differently in males (Sxl-m) versus females (Sxl-f) [245] and ultimately controls the gene doublesex (dsx) [121,526], which also has two isoforms (dsx-m vs. dsx-f) [448,1604]. Herein lies the most startling aspect of the human-fly affinity: we use a homolog of the same gene to regulate our dimorphisms (Dmrt) [1200,1378,1957]! Thus, the involvement of dsx in sex determination must predate the divergence of chordates, arthropods, and nematodes (see text). Fig. 5.1R We know a lot more about the genetics of sex determination in flies than in humans, thanks to decades of ingenious research—one of the most engrossing detective stories in the history of science [467]. Nevertheless, there is not a single sex-assignment pathway in any species that makes sense from the standpoint of efficiency [2064,2814]. Every cascade is more complex than it needs to be [2815], especially considering that all they really need is one switch [1172]. Of course, the same accusation could be leveled at lots of gender-neutral signaling pathways as well [1137]. (EGFR comes to mind [1137].) Baroque control systems like these epitomize the layering of evolutionary tinkering [226,683,1265]. They look as if they were wired by a succession of schizophrenic electricians who insisted on "improving" the shambles they found when they arrived. Kooky examples—don’t laugh!—include co-opting (1) a catenin to regulate transcription for the Wnt pathway [194,1015] and (2) a kinesin to relay signals for the Hh pathway [749], not to mention all the phosphatases that were recruited to undo what the last bloody kinase did and vice versa [1231,1232]. Indeed, our genome has even more dumb quirks than our body! In a pinch, what matters for evolution is expediency, not efficiency! As for why dimorphisms evolved from an ecological perspective, see ref. [2370]. a. One elusive piece of the dimorphism puzzle concerns our brain (item #5 on both lists in the figure). How different are men and women mentally, and to what extent are the disparities hardwired genetically [161]? In mice, surprisingly, the brain’s default state appears to be male, not female (as has been assumed for the body as a whole [221]) [2442]. In flies, much progress has been made in dissecting courtship behavior [197,2175,2375,2891] and male combat [660], and those findings might have some relevance to us, given that (1) we share dsx/Dmrt as a regulator [2273], and (2) we (like flies) express some direct, cell-autonomous (hormone-independent) effects of our XX versus XY constitution within our brains [649] (cf. marsupials [2157]), notwithstanding our reliance on gonadal hormones [221]. The virtue of studying fly (vs. human) neurobiology is that some clever experiments can be done. For example, when dimorphic neurons were placed under the control of a light trigger, the researchers could turn the male’s courtship song ON or OFF with the flip of a switch and thus localize the seranade circuit within the brain [470,673,2892]! See Fig. 6.1 for the source of the human drawings. (Apologies to the artist for doctoring them here!) b. The 25% difference in body size (female > male) is omitted in this "fly-fishing" diagram [537]. The male-specific muscle in flies can actually be formed from female cells [1385] because it is induced by a neighboring motor neuron whose gender is the deciding factor. The capture of the distalmost t-row by dsx evidently occurred via a new link between dsx and Scr [135]—viz., a de novo sex-specific regulation of the Hox selector gene Scr (Sex combs reduced) [1997], which governs the foreleg-bearing body segment [134]. How the enslaved t-row became a comb is actively being researched [1007,2124,2630]. The homology of dsx in flies with Dmrt in humans is startling. For a survey of similar homologies, consult the Interactive Fly. Bristle maps are adapted from ref. [1137]. Fig. 5.2 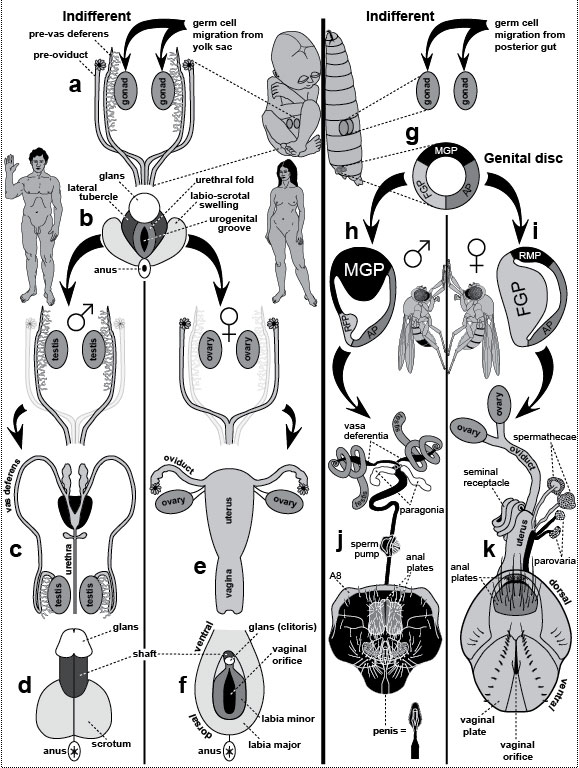 Genital development in humans (left) versus fruit flies (right). In both species the incipient genitalia look alike in males and females ("indifferent"), and the same goes for the gonads. Bilaterian gonads typically import their germ cells [923,1393,1453] after the latter have migrated far (using conserved genetic circuitry [2640]), a strange odyssey that may have a simple evo-devo explanation [630,736,737,2623]. Most important, each gender initiates some primordia appropriate for the opposite sex but then either destroys them (humans) or represses them (flies). Anatomy was redrawn from refs. [302,303,1866], and fate maps were derived from refs. [429,2270]. Genital development in humans (left) versus fruit flies (right). In both species the incipient genitalia look alike in males and females ("indifferent"), and the same goes for the gonads. Bilaterian gonads typically import their germ cells [923,1393,1453] after the latter have migrated far (using conserved genetic circuitry [2640]), a strange odyssey that may have a simple evo-devo explanation [630,736,737,2623]. Most important, each gender initiates some primordia appropriate for the opposite sex but then either destroys them (humans) or represses them (flies). Anatomy was redrawn from refs. [302,303,1866], and fate maps were derived from refs. [429,2270].a-f. Development of human genitalia [1866]. a. Internal genitalia in ~10-week embryo (of either sex). Two pairs of tubes flank the gonads. Pre-vasa deferentia (a.k.a. Wolffian ducts) have side branches (mesonephric tubules) medially, whereas pre-oviducts (a.k.a. Müllerian ducts) do not. All tubes converge on the urogenital sinus (not shown), which contains two more rudiments with divergent fates: one forms the prostate (male) or Skene’s gland (female) and another becomes Cowper’s (male) or Bartholin’s gland (female). b. External genitalia in ~10-week embryo (of either gender). Parts are coded by different shading to mark their homologies in males (d) versus females (f). c. Pre-oviducts disintegrate in males, leaving pre-vasa deferentia. Vasa lengthen as the testis descends into the scrotum before birth [1866]. Black organ at the juncture of vasa and urethra is the prostate. Paired glands above are seminal vesicles; paired glands below are bulbourethral (Cowper’s) glands. d. External genitalia in neonate. e. Pre-vasa deferentia disintegrate in females, leaving pre-oviducts. Ligaments connect the ovaries to the uterus [1866]. f. External genitalia in neonate (urethral opening omitted). Note the homologies: (1) penis ≈ clitoris and (2) scrotum ≈ labia major. g-k. Development of D. melanogaster genitalia [302,303]. g. Fly genitalia come from the genital "disc" [733,948], a midline rudiment that grows during the larval period and differentiates during metamorphosis (like the other 18 imaginal discs [1137]). It has 3 distinct zones: (1) a male genital primordium (MGP), (2) a female genital primordium (FGP), and (3) an anlage for anal plates (AP) [2270]. Each zone comes from a different body segment [429]. Zones are coded by different shading to track their fates in males (j) versus females (k). h. In males, growth of the FGP is repressed (RFP), but repression is incomplete because RFP still manages to contribute a thin eighth tergite (A8) to the cuticle (j) [2690]. j. The only part of the male made by RFP is a tiny collar (A8) around the genitals proper [429,1359,2690]. Paragonia come from cells recruited from outside the genital disc [28]. Testes change from oval to spiral shape after contact with vasa (cf. Fig. 2.1 for chirality) [2467,2468], but inductive signals have not yet been identified. Because the penis is difficult to discern amid all the clutter, it is rendered alongside as well. Insect genitalia evolve rapidly and are important taxonomically [697,1329,1425,2554,2793], so genital evolution has become a field unto itself [696,698,699,1220]—albeit one that is ill suited for polite discourse in mixed company. k. The only part of the female made by RMP is a stripe of the uterus and the two parovaria [1359,2690]. In females, anal plates (darker gray AP derivative) are oriented one above the other with the anus in between, whereas in males (j), they are oriented side by side. Fig. 5.2R The dorsal-ventral axis is reversed in these diagrams of humans (d, f) versus flies (j, k). The former show humans on their backs, and the latter show standing flies from the rear. d, f. Evidence of male-female genital homology is provided most starkly by genital masculinization in female hyenas [802,921,2869], an unfortunate trait that makes giving birth through the enlarged clitoris (≈penis) [803] as difficult as "pushing a golf ball through a soda straw" [1749]. On the basis of this oddity, Aristotle thought that female hyenas were actually hermaphrodites (History of Animals, Book 6, Part 32). A hotly disputed issue in evo-devo (what an understatement!) is whether human female orgasm is merely a spandrel [972,1209,1318,1609,2734]. (Don’t try debating this at home with your spouse!) The most exasperating aspect of spandrel debates in general is that they can involve untestable hypotheses [77,330,2105,2424], but meaningful questions can be posed and evaluated with the proper reasoning [2038]. j. Flies may have evolved elongated testes to fit longer sperm [2818], which reduce the chance that eggs will be fertilized by a subsequent suitor’s sperm [205,1994]. Indeed, runaway selection in one fly species (D. bifurca) has produced the longest sperm of any animal on earth (~6 cm) [2043]! The number of gyres per spiral (N) in Drosophila testes is only 2.5 for D. melanogaster [2604], but it is 6 for D. virilis, ~11 for D. hydei [2818], and an incredible ~40 for bifurca [2043]. N is correlated with the time available for testis elongation (t) [2467]. For a fixed growth rate "r," evolution could have easily modified N indirectly by changing t, given that N = rt [2781]. Similar logic applies to other spirals (e.g., mollusk shells, mammal horns, human cochlea [510,2133,2498,2596]). Indeed, heterochronic changes may have catalyzed the evolution of many other anatomical shapes as well [1709,1710]. Fig. 5.3 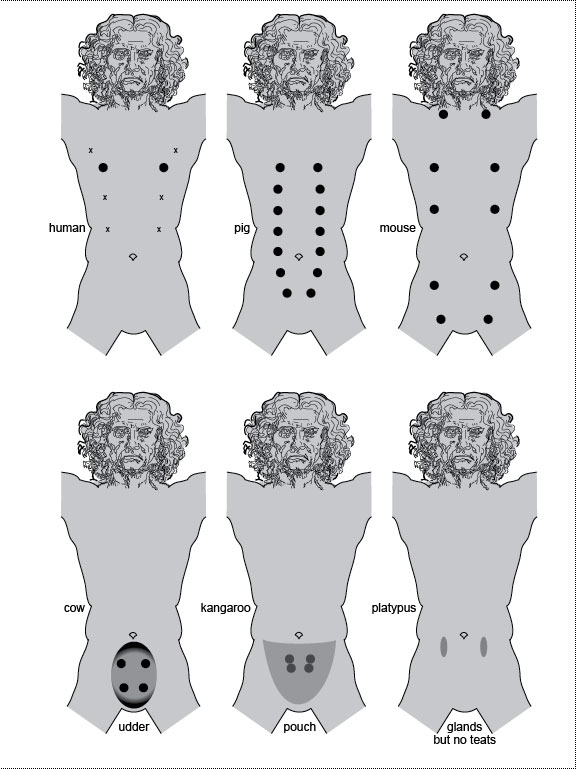 Mammary gland locations in various mammals. After Turner [2649]. Xs on the torso labeled "human" denote extra nipples in a German soldier (22-year-old male) [2802]. Similar atavisms were cited by Darwin [564], catalogued by Bateson [151], and described by others [187,408,2178,2431]. Most ectopic nipples in humans lie along these same "milk lines" [1023,2683], which stretch from the armpits to the groin [2198,2757]. In one grotesque case, a Thai woman had four big breasts, the extra two growing from her armpits [425]. Monotremes (platypus and echidna) have no nipples [2802]: their young lick the fur over the mammary glands [69,293], and the glands are nearly as well developed in males as in females [2802]. Mammary gland locations in various mammals. After Turner [2649]. Xs on the torso labeled "human" denote extra nipples in a German soldier (22-year-old male) [2802]. Similar atavisms were cited by Darwin [564], catalogued by Bateson [151], and described by others [187,408,2178,2431]. Most ectopic nipples in humans lie along these same "milk lines" [1023,2683], which stretch from the armpits to the groin [2198,2757]. In one grotesque case, a Thai woman had four big breasts, the extra two growing from her armpits [425]. Monotremes (platypus and echidna) have no nipples [2802]: their young lick the fur over the mammary glands [69,293], and the glands are nearly as well developed in males as in females [2802]. In marsupials, male neonates have fewer mammae than females [2158], or, in the case of Australian marsupials, no mammae at all [2197]. Male mice lack nipples (as do horses [69]) because they regress in response to androgens [687,2757] (but cf. [1539]). Other oddities (not shown) include (1) whales, which have two nipples straddling their genital opening [2051,2797]; (2) manatees, which have a nipple in each armpit [188]; and (3) the Virginia opossum, which has one median nipple ringed by 12 others [758]. The world record for teat number is the pouchless didelphia, Peramya henseli, which has up to 13 pairs [69]. Fig. 5.3R What is so surprising about mammary glands from an evo-devo standpoint is how early they develop in the embryo [91], considering that they aren’t needed until after puberty [2650]. Aristotle, like Darwin, wondered why males should have nipples {PoA:4:10:688b31ff} [137]: "In man there are breasts in the male as well as in the female; but some of the males of other animals are without them. Such, for instance, is the case with horses, some stallions being destitute of these parts, while others that resemble their dams have them." Unlike Darwin, however, he did not venture a guess as to why this is so. top of page
Lewis I. Held, Jr. is Associate Professor in the Department of Biology at Texas Tech University.
|
 © 2009 Thomas B. Brody, Ph.D. |