Select image to enlarge
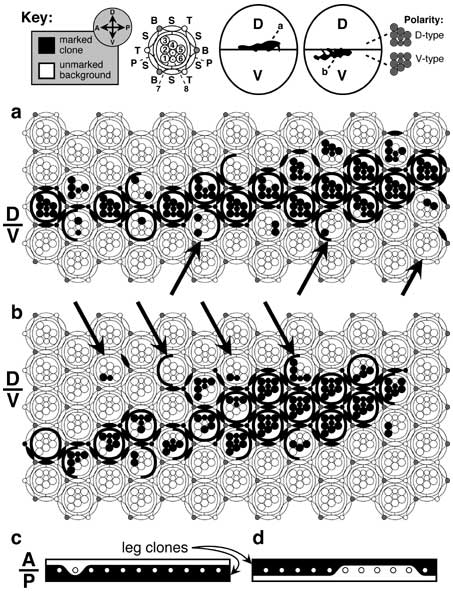
Figure 7.2
Lineage restrictions at the eye's equator. Until recently, the eye disc was thought to lack a D/V compartment boundary because clones can cross the equator. However, such transgressions are trivial (a, b), and similar trespassing occurs at bona fide compartment lines in other discs (c, d). In both cases, the 'noise' is attributable to patterning events that are superimposed upon -- but only loosely linked to -- the boundary. The key (top) shows (left to right): clone vs. background (black/white) markings, axes (D-V, dorsal-ventral; A-P, anterior-posterior), ommatidial template (B = 5-cell bristle organ; P, S, T = primary, secondary, and tertiary pigment cells; 1-8 = photoreceptor R1-R8 cells), conjectured locations of clones (enlarged in a and b; actual locations were not reported) within their respective eyes (ovals), trapezoid orientations in the D vs. V halves of the eye. Within the lattice the ommatidial 'repeat unit' has 23 cells -- counting each bristle as 5 cells and excluding the cells that each ommatidium shares with its neighbors [3539].
a, b. Clones of cells (black) that are homozygous for whiteLOF as a result of somatic recombination induced by X-rays in late 1st-instar heterozygotes [3539] when the eye rudiment has ~20 cells. Since an adult eye has ~750 ommatidia and only one of the two cross-over segregants becomes homozygous [1695], a single cell that undergoes recombination should leave marked descendants in 750 x 1/20 x 1/2 ≈ 20 ommatidia. Indeed, each of these clones covers ~20 ommatidia, but most are mosaic. The mosaicism shows that the ommatidium is not a clone. In the living fly, each clone was a ragged white stripe across an otherwise red (left) eye (cf. key). Except for bristles (shaded) all of the cell types shown are scorable phenotypically. The equator (D/V line at left) is defined by the symmetry planes of the columns (13 columns per eye are shown). The R1-R8 trapezoids in each column always point dorsally above a certain level and ventrally below it [2794], so that no D-type ommatidia ever get 'stranded' in the V half or vice versa [4797]. The equator zigzags through the lattice, jogging by one ommatidium from column to column. Normally it alternates -- jogging up then down (sawtooth mode) -- but in places it jogs one or two rows in the same direction (staircase mode) before returning to the baseline [2794, 3539, 4715]. a. Clone that evidently originated in the D half but which includes a few cells in 3 adjacent V-type ommatidia (arrows). b. Clone that must have arisen in the V half but which includes some cells in 4 D-type ommatidia (arrows). All but one of these 7 ommatidia (rightmost arrow in b) have a majority of their cells from the 'home' compartment, and even the exceptional case might be likewise since its other cells (e.g., cone cells; not shown; cf. Fig. 7.1) could be of 'home' provenance. Thus, it is unclear whether either clone violates the rule that 'ommatidia adopt a polarity (D- vs. V-type) consistent with the majority of their constituent cells'. This same trend is seen in Minute mosaics where the marked clones (induced at various ages) have a growth advantage [633].
c, d. Clones (black) marked with yellowLOF on two left 2nd-leg basitarsi (proximal-distal axis is left-to-right). Only one row of 11 bristles (circles) -- row 1 -- is depicted. This row resides at the A/P compartment boundary. Its bristles can come from either compartment, depending upon the individual leg. c. All bristles -- except the second one -- must have arisen from P cells because they are embraced by a clone of P provenance. d. Several of these same bristles must have arisen from A cells because they are in a clone of A provenance. Such vagaries probably reflect the stochastic way that SOPs are selected in proneural fields (cf. Fig. 3.9).
Panels a and b are redrawn from [3539]; panels c and d are redrawn from [1800] (6th leg in his Fig. 3b and 4th leg in Fig. 3a). In the key, the locations of the clones along the A-P axis (arbitrarily sited here) was not actually reported in the original paper. In c and d the contour of the clone boundary through the background epidermis (drawn straight) is not actually known because the yellowLOF mutation does not affect ordinary cuticle.
N.B.: Clone edges do not look this ragged in 3rd-instar discs -- especially near the D/V border [696]. The choppiness seen here in the adult is due, at least in part, to 90° rotation of ommatidial clusters in late 3rd instar (cf. Fig. 7.5a-c), which must detach closely related cells from one another at cluster perimeters by breaking junctions. There is no A/P compartment boundary in the eye itself, which lies entirely in the A region (cf. Fig. 7.1) [4146].
|
|
