by Lewis I. Held, Jr.
purchasing information
| Quirks of Human Anatomy by Lewis I. Held, Jr. |  purchasing information |
| back to Quirks index page | |
Figure Legends 3 3.1 * 3.1R * 3.2 * 3.2R figure legends 1 * 2 * 4 * 5 * 6 * 7 * A N.B.: An 'R' suffix denotes reflections (commentaries, annotations, and further references) pertaining to the numbered legend that precedes it. [Select any image to enlarge; use back button to return] Fig. 3.1 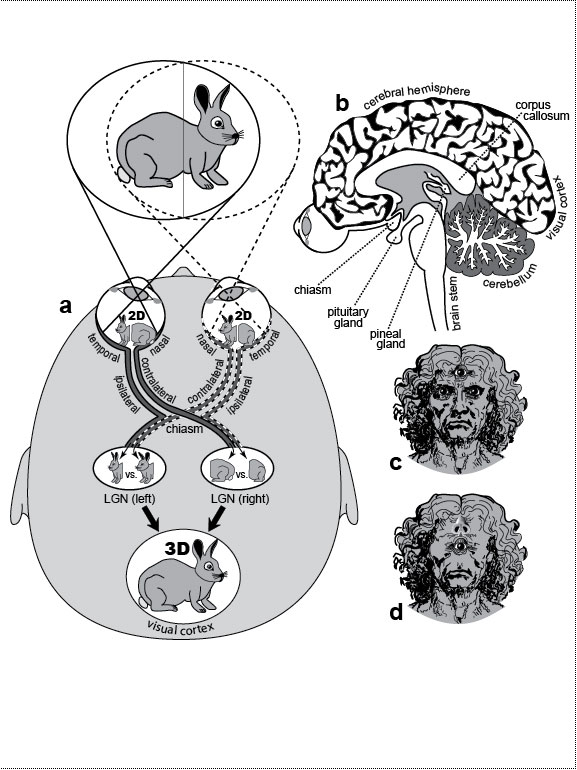 How our eyes perceive depth, and how we might look if we reverted to a 3-eyed (reptile) or 1-eyed (protochordate) stage of evolution. How our eyes perceive depth, and how we might look if we reverted to a 3-eyed (reptile) or 1-eyed (protochordate) stage of evolution.
a. Our eyes point frontally, and their fields overlap extensively. When we look at something like a rabbit, each eye sees it from a distinct angle. With the rabbit nearby and facing right, our left eye sees more of its rump, while our right eye sees more of its face (although the disparities are grossly exaggerated). Our lenses focus the inverted images onto our retinas, and each image is then bisected. "Nasal" axons (those near the nose) cross over (at the chiasm) to the lateral geniculate nucleus (LGN) on the contralateral (opposite) side, whereas "temporal" axons (those near the temple) go to the LGN on the ipsilateral (same) side. The half-images are stitched back together in our visual cortex [691], and depths are computed from differences between left and right images [2651,2783]. The farther away the rabbit, the more we rely on parallax disparities of the background (vs. those of the rabbit itself) [1037]. It is a testament to the virtuosity of our visual system that we are oblivious to our visual field being cut in half [6,647]. b. Sagittal section showing the visual pathway and pineal gland relative to other brain structures. c. What we might look like if our pineal gland regained its ability to form a third eye, assuming it could somehow grow through our cortex and skull (cf. [694]). Our fish ancestors may have actually had two such eyes at the midline [496]. The extra eye should function adequately because surgically implanted third eyes can weave their nerves into existing pathways [504,1895]. How a median eye might steer its axons at the chiasm, however, is unclear [752]. d. Holoprosencephaly (cyclops) syndrome in its most extreme manifestation. The nose lies above the eye, perhaps because the neural crest cells that normally migrate down between the eyes to make the nose can’t do so because they’re blocked by the median eye [2573,2811]. However, the culprit could instead be (1) absence of instructive signals from the underlying (abnormal) brain [712] or (2) blockage of other (permissive?) tissue shifts (T. C. Lacalli, personal communication). Fig. 3.1R a. Neural yoking devices force our eyes to (1) move together [767,1562], (2) blink together [2824], and even (3) constrict their pupils together [1588,2671,2810], although only the first reflex has any obvious utility—viz. the aiming of both foveae on the same objects for maximal acuity [2287] and depth perception [1147,2026,2452]. The cleavage plane that splits our visual field is shifted in albinos because of a defective tyrosinase enzyme that misroutes axons at the chiasm [1027,1282]. To compensate, albinos cross their eyes [67]. A similar syndrome affects Siamese cats [1323,2306] due to a milder mutation in the same gene [1912]. Interestingly, their black nose, ears, paws, and tail are also due to the disabled tyrosinase, which only makes pigment at the colder tips [2447]. Amazingly, a "Siamese cat" mutation has even been found in humans [2614]. Our eyes arise on the sides of our head like a fish and gradually move frontally [1998], an obvious validation of the largely discredited dictum that "ontogeny recapitulates phylogeny" [956]. At 6 weeks postconception, they point 160 degrees relative to one another (no binocular overlap). By 7 weeks, the interocular angle is ~120 degrees, and by 10 weeks, it is down to ~70 degrees, nearly reaching the adult angle of ~60 degrees [1998]. In contrast to vertebrates, arthropods only process their visual inputs ipsilaterally, a strategy more sparing of axon length. Nevertheless, they have their own quirky (unilateral) chiasms [312,2681]: the visual image gets inverted twice on its way back into the brain (180 degrees from lamina to medulla and another 180 degrees from medulla to lobula), thus restoring its original orientation [1946,1948]—a seemingly pointless exercise [1723]. b. Expression of Pax6 in the mouse pineal gland is further evidence for its homology with the lateral eyes [2030,2740]. The pituitary is another midline gland with at least as many plot twists in its history as the pineal [599], if not more of them [420,1350]. During human development, its front and rear halves fuse from separate parts that travel weird paths [1348,2192], and these parts are traceable to separate precursors in our pre-chordate [356,1191,1637,2361] and bilaterian [2588] ancestors. c. In hindsight, it might have been better if we had evolved the proverbial extra "eye(s) in the back of our head" [975]. Regardless of whether the pineal rudiment becomes an eye or a gland, it arises like an optic vesicle (cf. Fig. 6.2) insofar as it starts as a diverticulum from the diencephalon [376,539,1801,2278]. Because the lenses of the lateral eyes come from invaginations of head ectoderm [1084], one might expect the lenses of pineal eyes to arise likewise, but they actually develop within the vesicle wall itself (in reptiles) [694]. This distinction may not mean much, however, because lateral eyes in various vertebrates can regenerate a lens (from the iris! [1020,1125,1126]) through an intraplanar route [1273,2350,2639]. d. The proboscis is actually more tubular than depicted here [485,486], presumably because its shape depends on signals from the underlying forebrain [700,712,1217,1642], which itself is deformed in the syndrome [2811]. Cyclopic babies typically do not survive beyond a week after birth [140,1047]. Cyclopia is also seen in the fused heads of conjoined twins (cf. Fig. 3.2g). Fig. 3.2 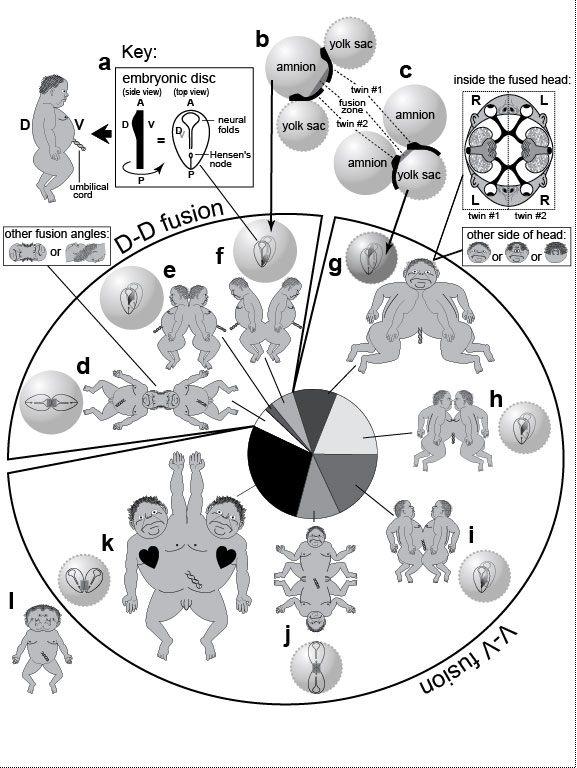 Extra planes of symmetry in conjoined twins [2435].
a. Axes of a normal embryo (A, anterior; P, posterior; D, dorsal; V, ventral). The human "embryonic disc" stage (~19 days after fertilization [1801]) is shaped like a guitar pick, as shown at the right, where the disc is seen D-side up (V-side dimmed below). The neural folds flanking the anterior neuropore (≈keyhole) will later meet at the midline to seal the roof of our brain [91]. Hensen’s node is where superficial cells went inside (to form the notochord) during the previous gastrulation stage. In the sketch at the left, the disc is drawn in side view as a bar that is swollen at the head end to denote the elevated neural folds there [1801,2311]. (The baby alongside is provided to clarify the D-V polarity.) This "swollen-bar" icon is used in b and c to represent the tail-to-tail (b) or head-to-head (c) orientation of adjacent twin embryos. Extra planes of symmetry in conjoined twins [2435].
a. Axes of a normal embryo (A, anterior; P, posterior; D, dorsal; V, ventral). The human "embryonic disc" stage (~19 days after fertilization [1801]) is shaped like a guitar pick, as shown at the right, where the disc is seen D-side up (V-side dimmed below). The neural folds flanking the anterior neuropore (≈keyhole) will later meet at the midline to seal the roof of our brain [91]. Hensen’s node is where superficial cells went inside (to form the notochord) during the previous gastrulation stage. In the sketch at the left, the disc is drawn in side view as a bar that is swollen at the head end to denote the elevated neural folds there [1801,2311]. (The baby alongside is provided to clarify the D-V polarity.) This "swollen-bar" icon is used in b and c to represent the tail-to-tail (b) or head-to-head (c) orientation of adjacent twin embryos.
b, c. Inferred geometries of fusing discs [2433]. >Each disc inflates two balloons made of spare embryonic tissues: an amnion dorsally and a yolk sac ventrally [91]. Depending on how the original zygote splits, the twins can either fuse dorsally ("D-D," d-f) across a shared amnion (b) or ventrally ("V-V," g-k) across a shared yolk sac (c) [1339], although in the latter case, the amnions must also fuse at a later time [1345]. Fusions are depicted as gray bands between the two embryos (black bars). There are eight categories of conjoined geometries, which occur at different frequencies (pie chart below; parasitic twins excluded) [2435]. d-f. Twins with two umbilical cords (13% total; yolk sacs omitted). d. Head fusions due to neural fold contact. Heads are often twisted at odd angles (inset), an asymmetry that rules out fission alone as the basis for joining. e. Back fusions due to medial contact. f. Sacral fusions due to posterior contact. g-l. Twins with one umbilical cord (87% total; amnions omitted) [278]. g. Frontal fusions due to contact of oral membranes [1152,1616]. The head has two faces that point sideways [1151,2436]. Structures often vanish at the fusion plane (small inset) [1616], resulting in cyclopia or fused ears [1345,2411]. Each face comes from the left half of one twin and the right half of the other, and the optic nerves detour to form inter-twin chiasms (large inset) [2688]. Darwin was fascinated with skewed fusions (small inset): "In one instance of two heads united almost face to face, but a little obliquely, four ears were developed, and on one side a perfect face, which was manifestly formed by the fusion of two half-faces" [560] (Vol. 2, p. 333). h. Thoracic fusions, in which the heart can have as many as seven chambers [911]. i. Abdominal fusions [1035], the most famous case of which were the original "Siamese" twins Chang and Eng Bunker (1811-1874) [678]. j. Posterior fusions, in which genitalia are diverted laterally like the half-faces in g. k. Side-to-side fusions with loss of tissue at the fusion plane, resulting in one pair of legs. The right twin often has situs inversus (heart on wrong side, etc.) due to trans-twin leakage of signals that stifle the nodal gene (cf. Fig. 2.3) [1529,2884]. Walking is difficult because of separate brain control [678,1751]. This Y-shaped anatomy (2 heads, 1 rear) can be artificially induced at high frequency (80%) in frogs by centrifuging fertilized eggs [214]. l. An anomaly not included in the pie chart is a single body with two heads (same etiology as k?) [348,911]. The heads can be fused, as sketched here for Lali Singh, a girl born in India in March 2008 (cf. Wikipedia: Diprosopus), or they can be separate, as for Syafirtri, a girl born in Indonesia in August 2006 (not shown; cf. Wikipedia: Polycephaly). Lali, who died two months after birth, was able to feed from either of her two mouths, and when she blinked, all four eyes blinked together. Fig. 3.2R Given that conjoined twinning has so little genetic basis [1991], any of us could have inadvertently sculpted a doppelgänger from part of our own body. Indeed, all vertebrate embryos are as malleable as putty at early stages [91,570]. This reality actually intruded into my brother’s family. His son Zachary had an identical twin who dueled with him in utero in a "Twin-Reversed Arterial Perfusion" Syndrome [2335]. When twins share a single placenta, one typically perfuses the other with venous blood [1073], dooming it to death at birth. Zack survived; his twin died. Indeed, half of such contests kill both twins [1631], so Zack was lucky to have survived at all. We are used to thinking of identical twins as being genetically identical, but this is certainly not true for females. Female mammals inactivate one X chromosome per cell during embryogenesis and hence are patchworks of paternal-X and maternal-X territories [1738]. Because inactivation occurs randomly (after the twinning stage), twin girls are unlikely to have same pattern of patches. Other epigenetic changes occur stochastically in both males and females after conception. Those changes include histone acetylation, DNA methylation, and DNA copy number variation—all of which must be taken into account in clinical twin studies [297,800]. As for how the splitting that causes twinning occurs in the first place, an early embryo might be bisected by a zone of cell death. An apt analogy may be the manner in which extra legs arise when parasites tunnel through the leg buds in frogs, creating fragments, each of which reorganizes "regulatively" [570] to form a whole leg [2339,2340]. Nevertheless, the splitting and rejoining of embryo fragments can also produce twins of unequal size [238,2434]—the hideous "parasitic twin" phenomenon [678,1524]. top of page
Lewis I. Held, Jr. is Associate Professor in the Department of Biology at Texas Tech University.
|
 © 2009 Thomas B. Brody, Ph.D. |